An Innovative Approach to Cancer Treatment: Click-Modified Exosomes Pave the Way for Targeted, Personalized Therapy
Cancer Treatment
Cancer research has reached a new frontier with a groundbreaking discovery that combines click chemistry with exosome technology, offering an innovative and potentially transformative way to treat cancer. Researchers, including Mr. Nobendu Mukerjee, Ms. Swastika Maitra, Dr. Nanasaheb D. Thorat, Dr. Prashant Kumar Singh, and Dr. Ajeet Kaushik, have revealed a method that modifies exosomes using click chemistry, a powerful tool that allows for precise modifications, greatly enhancing the targeting and delivery of therapeutic agents in cancer therapy.
What Are Exosomes and Why Are They Important?
Exosomes are tiny extracellular vesicles, typically ranging from 30 to 150 nanometers in diameter. These natural nanoparticles are secreted by nearly every type of cell, including cancer cells. They play an essential role in cell communication, transferring proteins, lipids, and genetic material like RNA. This unique ability to carry and protect biological molecules makes exosomes ideal candidates for therapeutic applications. In cancer treatment, they have shown promise as natural carriers for drugs, RNA molecules, and even gene-editing tools like CRISPR-Cas9. However, despite their potential, exosomes have limitations. Their heterogeneity (meaning that exosomes from different cells or even within the same tumor vary in composition) and instability in the bloodstream hinder their widespread use in cancer therapy. Traditional exosome-based treatments also face challenges such as poor targeting precision and limited drug-loading capacity.
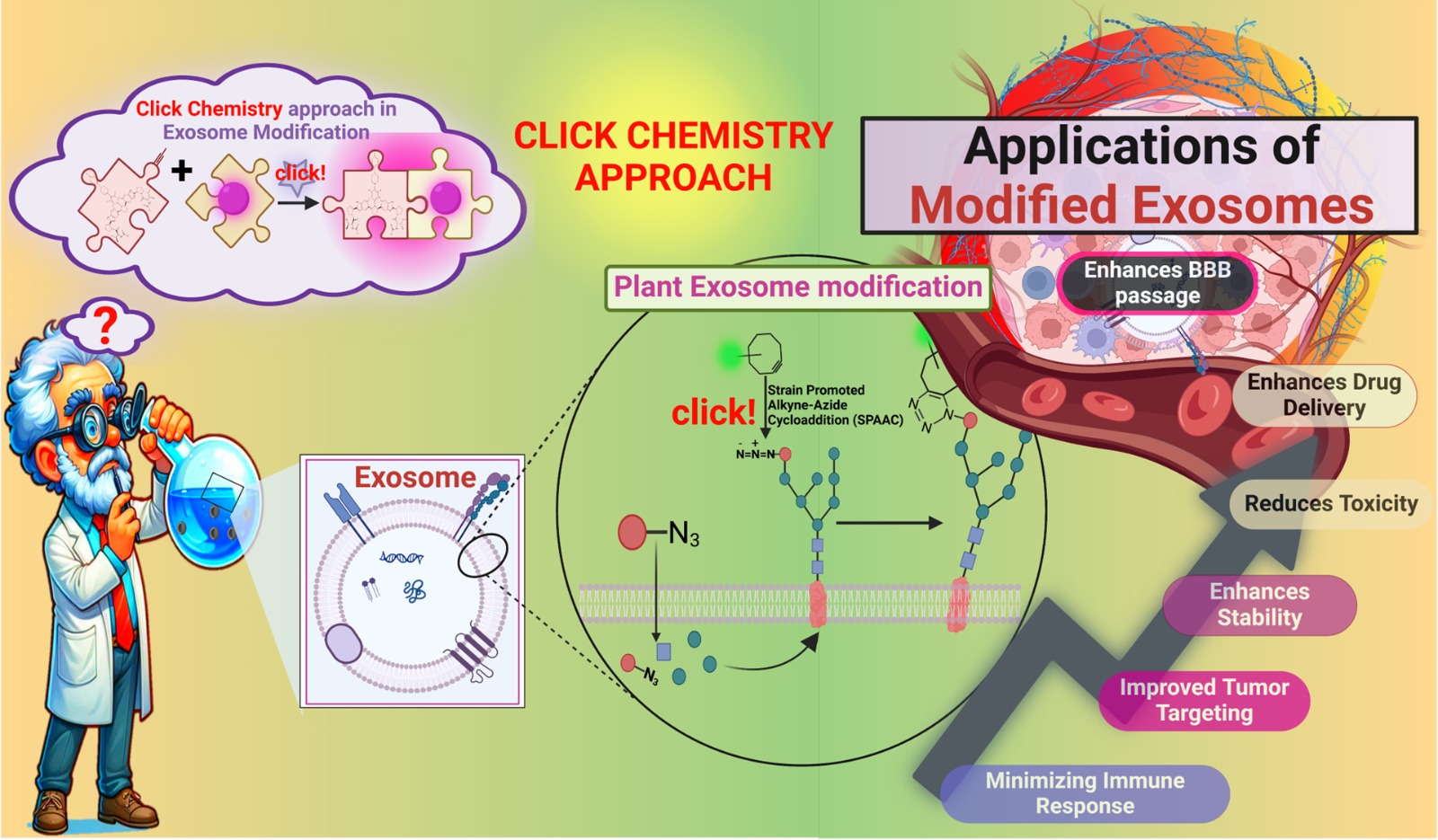
The Game-Changing Role of Modified Exosomes
Enter click chemistry, a set of chemical reactions that allows for highly selective and efficient modifications to biological molecules. This approach provides a way to chemically attach molecules, such as drugs or antibodies, to exosomes with remarkable precision and stability. The two primary reactions used are copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) and strain-promoted azide-alkyne cycloaddition (SPAAC), both of which enable the secure attachment of therapeutic molecules to exosomes without disrupting their natural functions. By utilizing click chemistry, researchers can engineer exosomes to be more effective drug delivery vehicles. This process involves attaching targeting ligands—such as antibodies or peptides—to the surface of exosomes, allowing them to home in on specific cancer cells, ensuring that the therapeutic agents are delivered directly to the tumor. This targeted approach minimizes the damage to healthy tissue, which is a common side effect of conventional cancer treatments. Moreover, exosomes modified with imaging agents can be tracked in real-time, allowing doctors to monitor the distribution and effectiveness of treatments. This combination of targeted delivery and diagnostic capabilities is called theranostics and holds great promise for improving cancer treatment, discussed in their newly published paper at Chemical Engineering Journal-Elsevier.
The Vision of the Research Team
The team behind this innovative approach includes Dr. Nobendu Mukerjee, Swastika Maitra, Dr. Nanasaheb D. Thorat, Dr. Prashant Kumar Singh, and Dr. Ajeet Kaushik, who have been working tirelessly to refine the process of exosome modification. Their primary goal is to enhance the targeting specificity, stability, and drug-loading capacity of exosomes to create more efficient and personalized cancer therapies. A major advancement from their research is the ability to increase the drug-loading capacity of exosomes. In traditional methods, exosomes have limited space to carry therapeutic agents, which restricts the number of drugs that can be delivered to cancer cells. By modifying the surface of exosomes using click chemistry, the team has been able to significantly increase their drug-carrying capacity. This allows for higher doses of chemotherapeutic agents, gene-editing tools, or RNA molecules to be delivered directly to the tumor site. Another breakthrough is addressing the challenge of exosome heterogeneity. By standardizing the modification process, the team has been able to create more uniform exosome populations, improving their therapeutic efficacy. These modifications also help to enhance the exosome’s ability to cross biological barriers, such as the blood-brain barrier, which is particularly important for treating brain cancers and neurological conditions.
Thanks to the visionary idea by the team: Dr. Nobendu Mukerjee, Swastika Maitra, Dr. Nanasaheb D. Thorat, Dr. Prashant Kumar Singh, and Dr. Ajeet Kaushik, the future of cancer therapy looks brighter than ever. Their idea into click chemistry-modified exosomes is a game-changer, offering the promise of more precise, effective, and personalized treatments. As clinical trials continue and further research unfolds, we may be on the brink of a new era in cancer therapy—one where exosomes take center stage in the fight against cancer and other diseases.






